How Does A Lead Acid Battery Work?
INTRODUCTION
Battery back up is essential in todays world, particularly when utilised in applications such as UPS systems and emergency lighting, but how does a lead acid battery produce the power needed to keep critical building services online?
There is a lot of information available on the internet which gives detailed information of the electrochemical reactions of the lead-acid battery. It can be seen that three effective components are require; lead, lead dioxide and dilute sulphuric acid. Looking at some of the basic information shows that Faraday discovered the theoretical amounts required to produce 1 ampere-hour (Ah) of electricity are; 3.87g of spongy lead (Pb), 4.46g of lead dioxide (PbO2) and 3.66g of dilute sulphuric acid (H2SO4). However, in practice many more time the theoretical value is required to produce an effective battery.
When a battery discharges it “uses” lead, lead dioxide and sulphuric acid. Eventually the battery will run out of one of these active materials and the voltage will collapse. It is not straightforward to say that all lead-acid batteries will run out of the same active material. Some will run out of positive active material, other negative and others acid.
This article will discuss how the three active materials work together to produce electrical energy. Basic battery designs are discussed along with the need to “design for manufacture”.
For this article we are only interested in industrial standby batteries on continuous float charge applications. However, some of the basic information is relevant to all lead-acid batteries.
POSITIVE ACTIVE MATERIAL - LEAD DIOXIDE (PbO2)
The positive active material is lead dioxide and this can be found in three basic plate designs; Planté, Pasted Plate and Tubular Plate. Some special designs are available but in reality all lead-acid batteries will have plates as described above.
In the Planté battery the plate consists of a lead casting upon which the active material is electro chemically formed. This type of battery is generally regarded as the most expensive to manufacture but it has a unique characteristic where the performance will improve by typically 10% over the first years in service before reaching a plateau. End of life is determined when the battery fails to achieve its nominal declared performance. This is typically after 25 years of float service. There are many examples of this battery type still in service after 40 years in service. For other battery types, the end of life is generally considered to be when 80% performance is reached.
Pasted Plate types are by far the most popular and can be found in VRLA batteries of the AGM and GEL construction as well as many vented types. The manufacturing process starts with lead oxide (PbO) as powder which is mixed with additives and dilute sulphuric acid to form a paste. This paste is applied to the lead grids.
Negative plates are manufactured in a similar way; starting with a lead powder, mixing with additives and dilute acid and applying as a paste to the lead grid. The negatives of Planté and Tubular Plate batteries are of the pasted plate type.
The plates are then formed either after assembly into the container or in large vats before being dried and then assembled into the battery.
For Tubular Positive Plates, the positive material it is often poured under pressure as slurry down the tubes. The material often includes triplumbic tetroxide (Pb3O4). This additive acts as a catalyst to assist the formation of the lead dioxide positive active material.
The performance and efficiency of the battery not only depends on the type (Planté, Pasted Plate or Tubular Plate) but also on the specific makeup of the material. Lead dioxide can be course where there is a lead (Pb) central core to the particles. In other types, the particle may be 100% lead oxide. Clearly, the more PbO2 and the more capacity can be expected – all other things being equal. Active material manufactured with triplumbic tetroxide additions is regarded as very efficient but has a manufacturing cost penalty.
Manufacturers have different ways of producing active material and it cannot be concluded that the battery having the heaviest weight will have the greater ampere-hour capacity.
NEGATIVE ACTIVE MATERIAL – LEAD (Pb)
The negative active material is a much simpler material than the positive. In all battery types, the negative plates are of the Pasted Plate type. The material is spongy lead (Pb) which chemically is basically the same as the base material; lead.
Particle size has little effect on the overall capacity of the battery and because it is much easier to form spongy lead when compared with the lead dioxide of the positive plate, the cost is lower. It is unusual that a battery performance is limited by the negative material.
ELECTROLYTE - DILUTE SULPHURIC ACID (H2SO4)
This is often ignored but without acid, the battery will not work.
The specific gravity plays an important part in the overall performance of the battery. Different types of battery use different concentration ranging typically from 1.210sg to 1.315sg.
Planté batteries have the greatest quantity of acid excepting special application batteries such as those designed with an extended topping up period. These special types start with a “high reserve” of acid which is generally of a quite low specific gravity (sg) such as 1.200sg. With time, the volume of electrolyte will reduce due to electrolysis and evaporation and the specific gravity will increase. Electrolysis consumes water and not acid. The volume of “acid” remains the same.
The strength of the electrolyte as sulphuric acid and water must be balanced to attain the correct strength for the type of battery. For example, if we start with 1.30sg acid and add water to reduce the strength to 1.20sg, the volume of “effective acid”, as determined by Faraday, remains the same. This is why we should refer to the electrolyte as dilute sulphuric acid and quote the specific gravity. It follows that if the available space in each cell is restricted, we must compensate by using a higher density (sg) electrolyte, i.e. we need more acid to balance the electrochemistry. We still need to balance the lead dioxide, lead and acid in the battery for it to work effectively.
The graph below gives an approximation between strength of acid and specific gravity (sg).
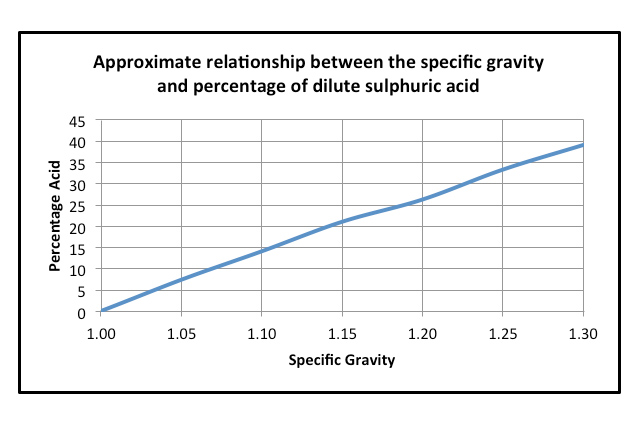
Because of the tight assembly of Tubular Plate batteries, there is very little space for the electrolyte and consequently the sg has to be higher; this is typically about 1.280 sg. Similarly VRLA AGM batteries have a higher sg, typically in the order of 1.315sg. These battery types generally run out of acid before they consume all the positive or negative active materials. They can be described as “electrolyte” starved. It may be argued that all we need to do is increase the strength of the electrolyte and stop these types from running out of acid. Unfortunately it is not that simple because very strong acid will destroy the negative active material.
VRLA GEL products generally use a lower density electrolyte than VRLA AGM because they have more space between the plates and room above the plates for electrolyte. On the down side GEL products do not have the same high current capability as AGM because the space between the plates is smaller. The reduction in space, known as plate pitch, means the resistance is lower and therefore the high current capability is better.
POSITIVE GRID ALLOY, DESIGN AND LIFE
The positive grids corrode as batteries age and this is often the cause of failure at the end of life. Several factors influence the corrosion rate of the positive grids including the grid alloy, design, thickness and method of manufacture.
In the past it has been argued that the thicker the grid, the longer it will last. This is no longer the case. Grids manufactured using pure lead of better than 99.99% purity, with life enhancing additives such as tin, silver and even gold, have been shown to last longer than grids many times thicker.
The method of manufacture is also critical. Expanded metal grids will corrode faster because of the “stress zones” where the metal is expanded. Arguably, rolled and punched grids will have the longest life. By rolling the grid metal the stress lines will lie in a uniform direction which makes them very corrosion resistance. This can be compared with cast iron and forges steel.
However, there is a cost penalty. Rolled and punched grids will cost more to manufacture when compared with cast grids or expanded metal grids. Similarly, very pure lead with expensive additives such as tin will again have a higher manufacturing cost.
NEGATIVE GRID ALLOY, DESIGN AND LIFE
Because the negative grids do not corrode in normal operation, the life of the battery is generally not cause by negative grid failure.
Designs are more simple, spongy lead is quite good at conducting the current and therefore the cost is much lower than that for the positive grid.
DESIGN FOR HIGH CURRENT APPLICATIONS
The high rate performance of a battery will be superior if a larger number of plates are used. Thinking of a loaf of bread, the thick slice “toast” bread may have 25 slices whereas the thin sliced loaf may have 35 slices, but the overall size will be the same. If this is translated to a battery technology, the more plates in the same volume will result is a greater high current capability. There is a down side to more plates. If the plates are thin, they are more difficult to handle during manufacture and therefore they will inevitably have a higher cost. Coupled with the number of plates is the plate pitch. i.e. the distance from the positive plate to the negative plate. The thinner the better but separators which are very thin are more difficult to handle and are more expensive.
The positive grid and to a lesser extent the negative grid design can also contribute to the high rate performance. The positive active material (PbO2) is not a particularly good conductor of electricity when compared with lead (Pb) of the negative plate and to compensate for this, the vertical wires and top bar may have an increased cross section as they approach the lug area. Designs with “vertical” wires radiating from the plate lugs (radial grids) are often used but again there is a cost in manufacture. Designs with increased sizes of wires will cost more to manufacture because the volume of lead and therefore the weight of the grids will be higher. Lead is the most expensive active component in a lead-acid battery.
The separator material also has an effect on the high rate performance. Separators with extremely low resistance are available but again there is a cost to be paid. The separator material for VRLA AGM batteries has a very low resistance and coupled with very small plate pitch, this makes them very good for high current applications.
DESIGN FOR LONG LIFE
Comparing designs for long life, you may think that the opposite applies to designs for high rate. This is not the case.
It has been shown that thin grids manufactured from very pure lead such as better than 99.99%, as discussed earlier in this paper, will enhance battery life. This also applies to the negative plate but to a much lower extent.
The purity of the filling acid can have a dramatic effect on life. This filling acid is made up of two parts; a) sulphuric acid and b) water to give the dilute sulphuric acid of the correct strength (sg) for the battery type.
The purity of the active material will also have an effect on life. Both positive and negative active materials come from base lead. It is also argued that virgin lead should be used if the longest life is expected. The best batteries will be manufactured from 99.99% pure lead or those where the critical impurities such as iron are kept to a very low level.
CONCLUSION
How a battery works is not simply down to the electrochemistry. The design, materials and manufacturing methods play a vital part in getting the best from the base materials discussed by Faraday.
Batteries manufactured using very pure materials, state of the are designs and manufacturing processes will offer the best return for long life, high performance and best reliability.
The market place has vented battery design such as Planté, Tubular Plate and Flat Plate designs to choose from. For VRLA types, the choice between AGM and GEL can be difficult but generally, the AGM battery will be smaller, lighter and have a better high rate performance. GEL batteries are not all the same and different life claims are seen for different designs even from the same manufacturer.
The choice of which battery to use is not easy and is even more complex when capital or whole life cost is thrown into the equation.
We need lead (Pb), lead dioxide (PbO2) and dilute sulphuric acid (H2SO4) for the battery to work but how we use these materials makes a substantial difference to the end product.
We hope our article has proved useful and informative, our next blog discussion will be ‘VRLA Battery Storage’ which will describe best practices to ensure VRLA batteries are stored correctly. In the meantime, please do contact us directly should you have any enquiries our team can assist you with.