How Should Industrial Batteries Be Stored?
This article discusses the effects of storing VRLA AGM & GEL types and also vented Planté, Pasted Plate and Tubular types.
The article covers the effects of time, temperature, humidity and light and also discusses sulphation; lead dendrite growth and corrosion of the internal lead parts as well as corrosion to the pillars and UV aging of the plastic containers. The relationship between open circuit voltage and state of charge is also discussed.
The article is intended to give the reader a better understanding to the reasons why battery manufacturers make specific recommendations for storing batteries which should not be ignored. It is not intended to be a definitive article and the battery manufacturer’s instructions should be followed at all times.
EFFECTS OF TIME
Battery manufacturers quote very different maximum storage times for their batteries and this can be confusing for the installer and user. One manufacturer may quote 3 months whilst another may quote 2 years. In the first instance we need to look at the storage temperature because the discrepancy in time may be explained by different temperatures. It is also important to confirm if the storage time is from the date of manufacture or first charge which is normally printed on the product, or from the date of dispatch from the manufacturing plant. Never the less, the storage time for “like to like” circumstances may be significantly different depending on many factors including design, materials and manufacturing differences.
Product quality in terms of material purity affects the storage time as does the minimum state of charge that the manufacturer is happy that the product may be successfully commission charged and what the commissioning charge recommendations are.
It is true to say that the longer the product has been stored; the more attention to commission charging is required.
For product supplied filled and nominally charged, the product will “self discharge” and lead sulphate will form on both positive and negative plates; and the specific gravity of the electrolyte will fall. The longer the storage time and the more difficult it is to convert this lead sulphate back to active materials. Where the product has been stored for a long period of time, some lead sulphate may never be re-converted and a permanent loss of capacity will result. Product that is supplied “dry charged” is more easily dealt with but this condition is reserved for vented Planté, vented Pasted Plate and vented Tubular types. Although the “dry charge” will be lost over the storage time, the condition can be recovered because the electrolyte is added after the storage time and only a limited amount of sulphate can be formed during storage. However, most manufacturers stipulate a storage time based on an 80% state of charge being achieved after filling with dilute sulphuric acid. A long storage time may result in no real capacity being seen after filling which can normally be recovered providing the correct charging regime is followed. This charging regime may be different than that required for a storage time within the manufacturer’s limits.
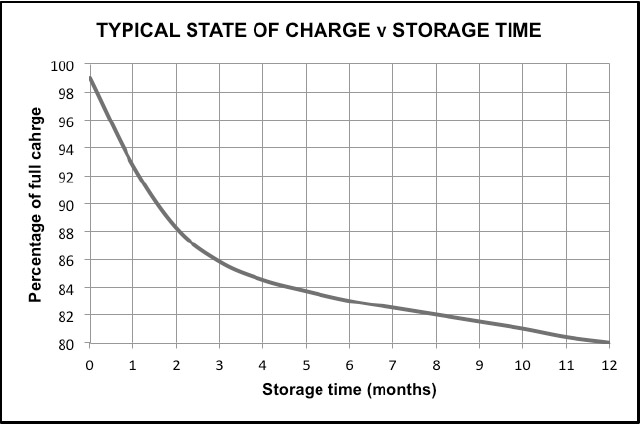
Fig 1 below shows the typical loss of capacity with time. This is an example only and should not be used to calculate the state of charge for any specific battery type. It can be seen that the capacity loss is more pronounced in the first months of storage before a more gradual fall off occurs.
EFFECTS OF TEMPERATURE
The correct storage temperature is arguably the most important aspect to consider. The higher the temperature and the quicker the capacity will fall and the shorter the recommended storage time.
Typically, manufacturers consider a storage temperature of 20ºC when quoting storage times. It is unwise to store a battery at above 40ºC for two main reasons: a) the storage time will be very short and b) at this temperature aging of the plastic components is accelerated. Whilst low temperatures will reduce the open circuit losses and give an extended storage time, it is normally recommended not to go below +5ºC. However, storage temperatures as low as -40ºC, and even below this for some types, are possible without problems providing the product is allowed to “thaw out” over several days until a more reasonable temperature above +5ºC is achieved before charging the battery. Very low temperatures will make plastic containers more brittle and special conditions may apply if the product is transported at these extreme temperatures.
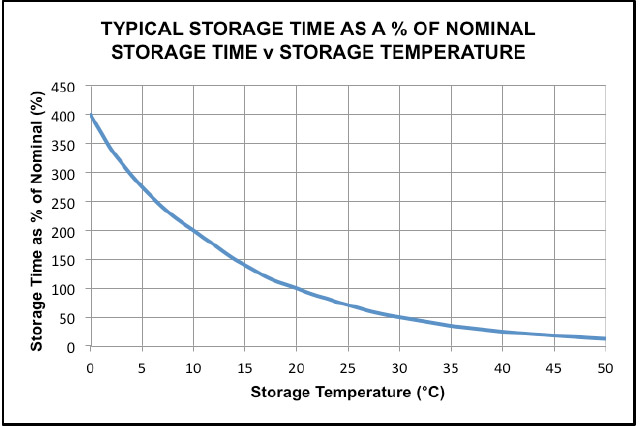
In Fig 2 below the storage time for different storage temperatures can be found. It can be seen that if the storage temperature is 20ºC, the storage time is shown as 100%. If the storage temperature is 30ºC, the graph shows 50% and if the manufacturer quotes a storage time of 18 months at 20°C the storage time at 30ºC becomes 9 months.. Similarly, if the storage temperature is 10ºC, the storage time will be 200%, i.e. 36 months. This graph is in the most part correct but it would be unwise to consider a storage time of 400% of the nominal for the reasons illustrated in the above section, “EFFECTS OF TIME”. Similarly, the maximum storage temperature should be considered as 40 ºC for the reasons given in this section above.
HUMIDITY
Storage humidity can have detrimental effects on the product. Terminal pillars are typically manufactured from lead alloys or brass and both will oxidize over time but this is accelerated in high humid environments. This corrosion should be removed prior to making the electrical connections. Failure to do this may lead to a high resistance joint and could, in extreme circumstances, lead to dramatic failures. The author of this document has firsthand experience of a battery fire developing as the direct result of severely oxidized terminals of a Planté battery where the terminals were not cleaned prior to installation. The storage time was less than 4 months but the humidity was extremely high.
INTERNAL CORROSION
Internal corrosion is completely unseen in VRLA product but can be considerable. In vented product assembled into transparent containers, “flaking” of the positive group bars and pillars inside the cells can become extensive. This may be severe enough to cause early battery failure. The “flakes” are not normally seen in storage but develop rapidly during the commissioning charge process. Typically, this is not noticed until the battery is several years old when failure due to internal shorts develops. To the experienced battery technician this type of failure can be clearly identified as a storage issue and not one of manufacture, over charging or undercharging. The structure of this corrosion is quite unique depending on how it was formed.
Corrosion to the plates is more subtle. Corrosion to the “weld” area between plates and group bars and group bars to pillars are the first to be affected. In some grid designs, the nodes between horizontal and vertical wires are affected. It is stressed that this corrosion is extremely rare and is insignificant if the product is stored correctly.
LEAD DENDRITES
When batteries are discharged the lead oxide (PbO2) of the positive plates and lead (Pb) of the negative plates are converted to lead sulphate (PbSO4). The extent that this occurs depends on the depth of discharge. The more rapid the discharge and the faster sulphate will be formed. When batteries are stored the self discharge is low but may be significant over time and this will be higher at elevated temperatures. Ultimately, lead sulphate will be formed and if the state of charge is very low, the specific gravity of the electrolyte will also be low and may be as low as 1.050 s.g. Under these circumstances, the lead sulphate (PbSO4) will react with the electrolyte (H2SO4) and some lead may go into solution. When the battery is eventually charged following storage, lead dendrites may be formed resulting in shorts between positive and negative plates.
Lead dendrites are rare because manufacturers often include chemicals to reduce their formation. Also, these lead dendrites only occur in VRLA AGM cells. Lead dendrites are not seen in vented products and VRLA GEL cells.
UV AGING
Battery containers can be affected by UV light. The effect is commonly known as UV degradation and sunlight contains a significant amount of UV light. The result of UV light can often be seen as cracking of the containers. Occasionally crazing of the container corners can be seen and a loss of colour definition can occur. Significantly many manufacturers include additive in the manufacture of containers to make them UV stable. However, this is very difficult where transparent containers are used such as with Planté and other vented products where clear containers are used.
It should be remembered that UV will affect the product once installed and direct sunlight on the battery should be eliminated.
OPEN CIRCUIT VOLTAGE
Within reasonable limits, there is a direct relationship between the open circuit voltage of a lead acid cell and the specific gravity of the electrolyte. For this relationship to be accurate, the cell must have been on open circuit for at least 12 hours and preferably 24 hours. It therefore follows that it will be reasonably accurate for product that has been in store for several days or more. The temperature limits for this technique are reasonably wide but for best accuracy this should be taken as +10°C to +30°C.
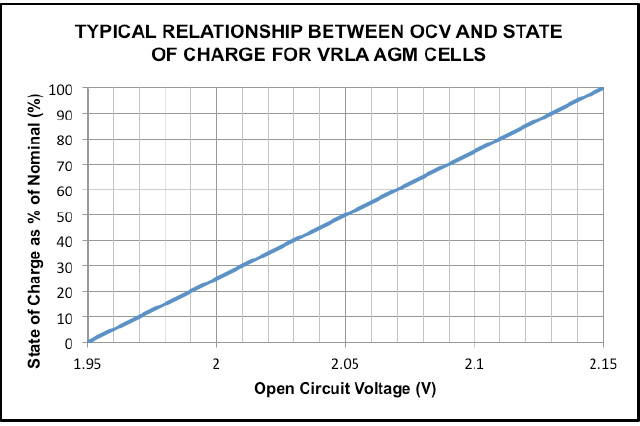
If we know the fully charged and fully discharged specific gravity of the cell then we can calculate the state of charge. This is illustrated below in Fig 3 for a typical VRLA AGM product.
The mathematical relationship is: - OCV – 0.84 = the s.g. of the electrolyte.
Example 1: OCV = 2.15V S.G. = 2.15 – 0.84 = 1.31 s.g..
Example 2: OCV = 1.95V S.G. = 1.95 – 0.84 = 1.11 s.g.
Note: Even at an open circuit of 2.00V, the cell will only be about 25% charged or, more to the point, 75% discharged.
A typical average rate of losses is 2% per month which equates to 3 years for a 75% discharged cell. Recovery from this very low state of charge is unlikely without a considerable capacity loss. A reasonable minimum open circuit voltage is typically about 2.10V for VRLA AGM cells. The product should be recharged if this voltage is reached because anything lower than this is likely to result in a permanent capacity loss. The 2.10Vpc represents about 75% charged.
The mathematical formula is relevant to all lead acid cells, not only VRLA types. However, the fully charged and fully discharged s.g. will be different for different battery types; particularly vented types compared with VRLA.
The fully charged specific gravity of Planté cells is typically in the order of 1.215, for Vented Pasted Plate types a value of about 1.250 is typical and for Vented Tubular Plate about 1.280. For vented cells it is more accurate to use a hydrometer or density meter to measure the specific gravity and from this the state of charge can be determined.
To make best use of this technique it is important that the fully charged and fully discharged specific gravity is known and at what reference temperature. For VRLA AGM cells where the voltage is measured and cross referenced to the state of charge the technique is reasonably accurate. This is because there is a large difference between the fully charged and fully discharged s.g.; typically 1.310 when fully charged to 1.110 when fully discharged.
CONCLUSIONS
The storage conditions can interact and cause serious problems. In particular, whilst the store time may be extended at lower temperatures, this time frame must not be extended beyond the recommended limit as advised by the manufacturer.
Where high storage temperatures such as above 30°C are experienced, consideration to the humidity is very important. High temperatures with high humidity will cause rapid corrosion to terminals and if this is not addressed, high resistance electrical connections may result.
In conclusion, always follow the manufactures instructions.